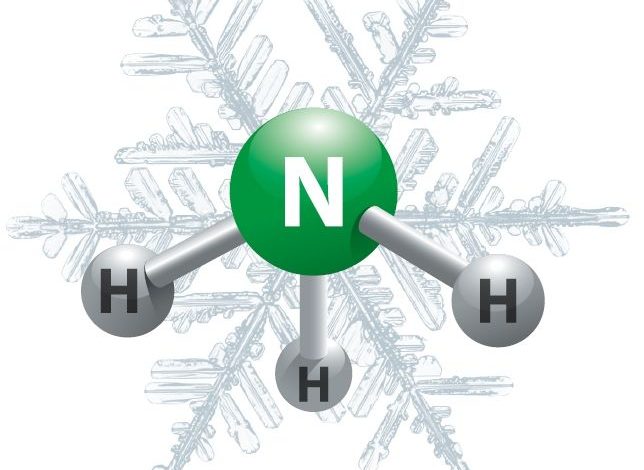
NH3 (Ammonia) electron calculation is “Tetrahedral” yet its sub-atomic math is “Three-sided Pyramidal”. The most ideal approach to sort this out is to draw the Lewis structure. For the NH3 Lewis structure, compute the absolute number of valence electrons for the NH3 atom (NH3 has 8 valence electrons, 5 from N and 3 from three H’s). In the wake of deciding the number of valence electrons there are in NH3, place them around the focal particle to finish the octets. Make certain to utilize the quantity of accessible valence electrons you discovered before. Hydrogen (H) iotas consistently go outwardly of a Lewis structure.
the H molecules are being moved somewhere near the electron pair on top and have an inexact bond point of 109.5 degree. This outcomes in a pyramid shape with N being at the pinnacle and three H’s on the corners.
NH3 (Ammonia) is an ordinarily tried Lewis structure. It’s not especially troublesome yet is a significant construction. Visit nh3 Lewis structure
In the NH3 Lewis construction (and all designs), hydrogen goes outwardly. Keep in mind that hydrogen just necessities two valence electrons to have a full external shell.
In the Lewis structure for NH3 there are a sum of 8 valence electrons. Three sets will be utilized in the synthetic connections between the N and H and one sets of electrons will be unbonded.
Nitrogen requires a solitary pair of electrons to finish the construction and give five electrons as suggested by nitrogen’s unique natural valence shell which contains similar number of electrons. It is hard to determine the specific 3D construction, however it follows an overall tetrahedron structure appeared underneath. Because of electron-electron repugnance in the solitary pair, the points between the leftover hydrogens is marginally under 109.5˚: 107.5˚. This spots nitrogen into the class of “three-sided pyramidal” mathematical designs.
Alkali is identified with ammonium (NH4+), whose Lewis structure is appeared here. Alkali is by and large a base. It is a kind of nitrogenous waste particularly predominant in oceanic life forms. It is viewed as a danger toward human wellbeing and can effect sly affect the neural framework as demonstrated in the graph beneath.
It is additionally one of only a handful few mixtures which can go through hydrogen holding because of its polar nature. Because of the strength of these hydrogen bonds, water has a moderately high liquefying and edge of boiling over, despite the fact that they are not as high as organization covalent solids. Those are fortified by intramolecular powers which include the genuine sharing of electrons versus incomplete dipole powers in hydrogen bonds. There are just three sorts of bonds which would hydrogen be able to bond. These are N-H, O-H, and F-H bonds because of the enormous electronegativity contrasts between the particles. In the H2O article, alkali was momentarily referenced as a compound with these properties. These properties add to NO3s moderately high edge of boiling over of – 33.3˚C and high dissolvability in water.
To comprehend the hybridization of smelling salts we need to painstakingly analyze the regions around Nitrogen. On the off chance that we take a gander at the nuclear number of nitrogen it is 7 and in the event that we consider its ground state it is given as 1s2, 2s2,2p3.
During the arrangement of alkali, one 2s orbital and three 2p orbitals of nitrogen join to shape four crossover orbitals having identical energy which is then considered as a sp3 sort of hybridization.
Further, on the off chance that we take a gander at the NH3 particle, you will see that the three half-filled sp3 orbitals of nitrogen structure bonds to hydrogen’s three iotas. Nonetheless, the fourth sp3 orbital that is available is a nonbonding pair of hybridized orbital and is ordinarily utilized for holding the solitary pair.
Significant Points To Remember
In NH3 hybridization, the three hydrogens will be based around the focal particle of nitrogen.
The hydrogen particles are simply s orbitals covering those sp3 orbitals.
NH3 Molecular Geometry And Bond Angles
In the event that we take a gander at the sub-atomic math of alkali it has a three-sided pyramidal or mutilated tetrahedral structure. This is predominantly because of the presence of a solitary non-holding pair which ordinarily applies more prominent shock on the holding orbitals. Taking all things together this, nitrogen lies at the middle, three hydrogen particles which are indistinguishable make the base and one sets of electrons frames the zenith of the pyramid. Visit Chemical Products Industry
The bond point in smelling salts is not exactly the standard 109.5o. The bond point is 107o.
The Lewis structure for smelling salts shows that there are four electron gatherings (3 single bonds and 1 solitary pair of electrons) in this way the electron bunch math is likewise tetrahedral. It ought to be noted anyway that CH4has 4 molecules attached to the focal particle, while NH3only has 3 iotas clung to the focal particle. Alkali thusly they won’t have a similar shape as CH4. Sub-atomic shape portrays the game plan of particles about the focal molecule. While deciding the sub-atomic shape, you should consider the electron bunch math and the quantity of iotas attached to the focal particle. (Solitary sets are overlooked now.) The potential blends of electron gatherings and reinforced iotas are summed up beneath.
After the calculations have been allotted to an atom, we choose if there is more than one right design for it. These right designs are called reverberation structures. Finally, we can utilize the sub-atomic shape to decide whether electron thickness is equitably disseminated across the particle. In the event that electron thickness is unevenly disseminated across the atom, the particle is supposed to be polar. A particle with a uniform charge dispersion is nonpolar. However, first you should figure out how to draw Lewis speck structures.